Anti-Aging Research: Kisspeptin
----------------------------------------------------------------------------------------------------------------------------------------------------------
*** SPECIAL NOTE FROM DR. WICHMAN ***
The following excellent article was reproduced from the Singapore Medical Journal at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4678402/:
Kisspeptin signalling and its roles in humans
Abstract
Kisspeptins are a group of peptide fragments encoded by the KISS1 gene in humans. They bind to kisspeptin receptors with equal efficacy. Kisspeptins and their receptors are expressed by neurons in the arcuate and anteroventral periventricular nuclei of the hypothalamus. Oestrogen mediates negative feedback of gonadotrophin-releasing hormone secretion via the arcuate nucleus. Conversely, it exerts positive feedback via the anteroventral periventricular nucleus. The sexual dimorphism of these nuclei accounts for the differential behaviour of the hypothalamic-pituitary-gonadal axis between genders. Kisspeptins are essential for reproductive function. Puberty is regulated by the maturation of kisspeptin neurons and by interactions between kisspeptins and leptin. Hence, kisspeptins have potential diagnostic and therapeutic applications. Kisspeptin agonists may be used to localise lesions in cases of hypothalamic-pituitary-gonadal axis dysfunction and evaluate the gonadotrophic potential of subfertile individuals. Kisspeptin antagonists may be useful as contraceptives in women, through the prevention of premature luteinisation during in vitro fertilisation, and in the treatment of sex steroid-dependent diseases and metastatic cancers.
INTRODUCTION
This review uses the nomenclature proposed by Gottsch et al to describe the kisspeptin signalling system. KISS1 and Kiss1 refer to the human and non-human genes for kisspeptins, while KISS1R and Kiss1r refer to the human and non-human receptors, respectively. The gene products of KISS1 and Kiss1 are collectively known as kisspeptins.(1)
The KISS1/Kiss1 gene encodes the kisspeptin precursor, a peptide comprising 145 amino acids.(2) This is proteolysed to fragments of various lengths.(3-5) Kisspeptin-54, comprising 54 amino acids, is the major fragment,(5) while other fragments include kisspeptin-10, kisspeptin-13 and kisspeptin-14. (Fig. 1) These fragments share an RFamide motif at the carboxy terminal.(3-5) Kisspeptins are expressed in the hypothalamus, gonads, placenta, liver and pancreas,(3-6) and bind to KISS1R/Kiss1r with equal efficacy.(3)
Kiss1r was discovered as an orphan receptor in the rat brain. It is a G-protein coupled receptor (GPCR) with homology to galanin receptors.(6) KISS1R was discovered later, and was variably termed GPR54, AXOR12, hOT7T175 and HH8.(3-5) KISS1R/Kiss1r was subsequently recognised as the endogenous kisspeptin receptor. It is expressed in the hypothalamus, pituitary gland, gonad, placenta, pancreas and kidney.(3,4,6-10)
INTRACELLULAR SIGNALLING MECHANISMS
KISS1R/Kiss1r is a GPCR coupled to the G protein subunit, Gq/11α(3,4) Ligand binding activates phospholipase C (PLC), leading to the hydrolysis of phosphatidyl inositol bisphosphate and the formation of diacylglycerol (DAG). DAG then activates protein kinase C. Mitogen-activated protein kinases (MAPKs) are phosphorylated, thereby activating β-arrestin and inositol-1,4,5-triphosphate. Consequently, intracellular calcium is released, depolarising the neuron(3,4,11-15) (Fig. 2). PLC-independent mechanisms may also increase the intracellular calcium level; these include the opening of inwardly rectifying potassium channels and non-selective cation channels.(13,16) Kisspeptin neurons form synapses with gonadotrophin-releasing hormone (GnRH) expressing neurons. Depolarisation of kisspeptin neurons leads to the depolarisation of GnRH neurons, and subsequent modulation of luteinising hormone (LH) and follicle-stimulating hormone (FSH) release.

KISS1R/Kiss1r is desensitised with continuous kisspeptin exposure. In animals, intermittent kisspeptin administration was found to raise LH levels. However, LH was suppressed after continuous administration of kisspeptin, as it induced long-lasting depolarisation in GnRH neurons, followed by desensitisation.(7,9,13,16-24) Such desensitisation is not due to GnRH depletion,(7,17) but may be due to clathrin-mediated internalisation of arrestin and KISS1R in internalised vesicles after kisspeptin activation.(15)
Cotransmitters of kisspeptin signalling
Neurokinin B (NKB) and dynorphin (Dyn) are cotransmitters of kisspeptin signalling. NKB is a tachykinin peptide that binds to the receptor NK3R.(25) In humans, NKB is called TAC3 and binds to the receptor TACR3.(27) Dyn is an endogenous opioid peptide that binds to the kappa opioid receptor.(28) NKB is an excitatory stimulus,(29-32) while Dyn is an inhibitory stimulus to kisspeptin release in the hypothalamus (Fig. 3).(28,30,33) Evidence of NKB and Dyn as cotransmitters includes the colocalisation of Kiss1, NKB and Dyn in the neurons of animal hypothalami.(34-36) These neurons are termed kisspeptin/NKB/Dyn (KNDy) neurons and project to GnRH neurons in animals,(34) in keeping with the role of kisspeptins in modulating GnRH release (Fig. 3). Similarly, NK3Rs are found in the hypothalami and GnRH neurons of animals.(37-39) Importantly, NK3R agonists activated Kiss1 neurons and increased LH secretion in rats. This observation was, however, absent in Kiss1r knockout mice,(29-31) suggesting that NKB is upstream of Kiss1r in the signalling pathway.(32) In addition, inactivating mutations of TAC3 and TACR3 cause hypogonadotrophic hypogonadism (HH).(27) Dyn was also found to inhibit LH secretion in animals.(28,30,33)
Neuroanatomy of the kisspeptin signalling system
Kisspeptins and their receptors are localised to various parts of the nervous system. The location of kisspeptin neurons differs between animal species. In humans, kisspeptin neurons were identified in the hypothalamus, basal ganglia and periventricular region, while KISS1R was localised to the hypothalamus, basal ganglia, amygdala, substantia nigra, hippocampus and spinal cord.(4,5,36) In rodents, kisspeptin neurons were demonstrated in various parts of the hypothalamus, including the arcuate nucleus (ARC) and the anteroventral periventricular nucleus (AVPV);(40-45) kisspeptin neurons in the AVPV formed the major afferent neurons to GnRH neurons.(42) In sheep hypothalami, kisspeptins were detected in the preoptic area (POA) and the ARC,(46-48) with the kisspeptin neurons in the POA forming the major afferent neurons to GnRH neurons.(47)
REPRODUCTIVE ROLE
Regulation of GnRH
Kisspeptin neurons are located in the posterior part of the ARC, which is the putative GnRH pulse generator in primates.(49) Administration of kisspeptin antagonists in this region suppresses GnRH pulsatility in animals,(50,51) suggesting that kisspeptin neurons form the GnRH pulse generator. Kisspeptins stimulate GnRH secretion by GnRH neurons. This effect was observed with both central and systemic administration of kisspeptins.(52-58) Human studies showed kisspeptin-induced LH release in women.(59) In animals, GnRH and LH levels are elevated by kisspeptins.(7,52,54,58,60-62) Kisspeptins also induced C-fos expression (marker of cellular activation) in rodent GnRH neurons(8,54) and evoked depolarisation of GnRH neurons in electrophysiological studies.(9,13,16,19,20) Moreover, the stimulatory effect of kisspeptins can be blocked by GnRH antagonists.(53,54,60,62,63) Human subjects with KISS1R mutations and HH responded to GnRH, while mice with Kiss1r mutations preserved hypothalamic GnRH content.(64) Collectively, these observations affirm that kisspeptins are excitatory stimuli located upstream of GnRH in the hypothalamic-pituitary-gonadal (HPG) axis.
It is unclear whether kisspeptins directly regulate pituitary function. In contrast to its effect on LH secretion, the FSH response to kisspeptin is comparatively delayed and less robust in humans and rats.(59,63,65) This may be due to differences in secretory patterns of the gonadotrophins,(66) different gonadotroph responses to kisspeptins(65,67) or different feedback signals to the gonadotrophs (e.g. inhibin).(68,69)
Sexual dimorphism
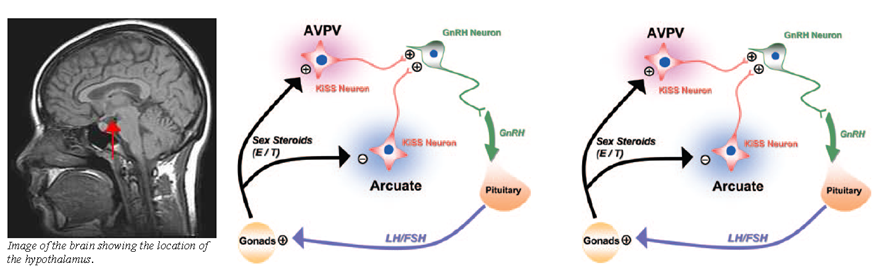
Kisspeptin neurons are located in two main regions of the hypothalamus. The first region is the ARC in rodents(44,45,70) or its equivalent, the infundibular nucleus, in primates.(71-73) This group of neurons mediates oestrogen-induced negative feedback on the HPG axis.(49,72,73) The second region is the AVPV in rodents, or the equivalent POA in sheep and primates.(25,36,74) This group of neurons mediates positive feedback from oestrogen(75-78) (Fig. 4). AVPV neurons behave differently between the genders. Only female AVPV neurons, which are also larger and more numerous,(43,70) demonstrate the LH surge in response to oestrogen.(79-83) Similarly, oestrogen increases Kiss1 mRNA and kisspeptin levels in rodent AVPV.(44,45)

Sex steroid exposure in utero may determine the behaviour of the kisspeptin system and HPG axis after sexual maturation. In rats, neonatal exposure to oestrogenic compounds suppressed kisspeptin production in the peripubertal and adult stages.(52,84,85) This resulted in HH, which resolved with exogenous kisspeptin. In neonatal female rats, exposure to androgenic compounds led to reduced kisspeptin production by the AVPV during adulthood. This was not reversible with exogenous oestrogen.(70) Thus, early androgen exposure led to androgenisation of the AVPV. Conversely, gonadectomised neonatal male rats had increased kisspeptin production in the AVPV during adulthood. These rats demonstrated the LH surge with exogenous oestrogen, indicating feminisation of the AVPV.(86)
Negative feedback regulation
Oestrogen and testosterone exert negative feedback on GnRH release (Fig. 4b), which is mediated via oestrogen receptor α (ERα).(44,45,52) Testosterone is aromatised to oestrogen prior to receptor binding(45, 87) (Fig. 4). However, GnRH neurons do not express ERα, while kisspeptin neurons do.(44,45,71,75,88) Hence, it is likely that oestrogen binds to ERα on kisspeptin neurons in the ARC or infundibular nucleus, inhibiting kisspeptin and subsequently GnRH release.(44) Several lines of evidence support this hypothesis. In gonadectomised animals and humans, sex steroid levels declined, while kisspeptins, GnRH and gonadotrophin levels increased.(8,44,45,52,71,72,89,90) However, antagonism of Kiss1r in rodents prevented the rise of gonadotrophins.(31,50,91,92) Notably, the elevated kisspeptin levels were localised to the ARC or infundibular nucleus.(44,45,52,71,73,87) Gonadectomised Kiss1r and Kiss1 knockout mice demonstrated similar behaviour.(31,91,92) Kisspeptin production in the ARC was shown to be reduced by sex steroid administration in animal studies.(44,52,70,87)
Positive feedback regulation
Oestrogen exerts positive feedback on kisspeptin neurons in the AVPV in animals. This effect is also mediated via ERα,(44,45,93,94) and may account for the LH surge in the menstrual cycle. The evidence supporting this includes the fall in kisspeptin level in gonadectomised animals, which was more pronounced in females.(44,45) Secondly, AVPV kisspeptin levels rose prior to the LH surge in animals;(41,95) pharmacologic studies confirmed that the LH surge was induced by kisspeptins.(96-98) Furthermore, LH levels increased with kisspeptin administration in women(99) and central infusion of kisspeptin antagonists blocked the LH surge in animals.(100,101) Thirdly, lesions in the AVPV prevented the LH surge, while oestrogen administration to the AVPV caused it.(96,102-105) Lastly, blockage of ERα prevented the LH surge in animals.(44,45,93,94)
Reproductive function and puberty
Kisspeptins assume a key role in reproduction. HH occurs in humans and mice with defective KISS1R and Kiss1r.(7,106-109)Notably, kisspeptins regulate the HPG axis by binding to Kiss1r in the hypothalamus, as evidenced by the failure of kisspeptins to increase LH or C-fos in the GnRH neurons of Kiss1r knockout mice.(7,110)
The maturation of kisspeptin neurons may be responsible for puberty, as suggested by the following. Firstly, inactivating mutations in KISS1R/Kiss1r,(106-108) and KISS1/Kiss1(64,111) led to HH and pubertal failure. Secondly, animal studies demonstrated an increase in Kiss1 and Kiss1r in the AVPV and/or ARC at puberty;(9,43,52,62,112-117) more kisspeptin neurons also project to GnRH neurons.(43,118-120) Thirdly, GnRH response to kisspeptins increases after puberty, which is secondary to improved signalling efficiency rather than increased receptor density(9) (Fig. 5). Finally, exogenous kisspeptins induced precocious puberty in rodents and monkeys,(9,54,62,121) and stimulated a GnRH secretion pattern resembling puberty,(22,122) while kisspeptin antagonists delayed puberty in these animals.(122,123)
Kisspeptins and energy homeostasis
Energy homeostasis and the reproductive system are linked, as exemplified by HH occurring in energy-deficient states. Energy-deprived rats and sheep had reduced Kiss1 mRNA in their hypothalami, reduced LH levels and pubertal arrest.(47,124-131) With kisspeptin administration, puberty resumed, while gonadotrophin and androgen levels normalised.(57,124,125) Interactions between kisspeptins and leptin may account for these observations. This postulation is supported by the finding that leptin activates GnRH neurons.(132) However, GnRH neurons lack leptin receptors, while kisspeptin neurons in the ARC express the leptin receptor gene.(47,133,134) Additionally, leptin-deficient rats have reduced Kiss1 mRNA in their ARC.(133,135) Leptin administration increased hypothalamic Kiss1 mRNA in fasted rats(125) and cell models.(129,136) It also depolarised kisspeptin neurons in the ARC of rats.(137) Therefore, it is likely that leptin activates GnRH neurons via stimulation of kisspeptin neurons in the ARC.
OTHER ROLES
Kisspeptin was initially discovered in 1996 as a metastasis suppressor(138) and was named metastin.(138,139) Over the last two decades, increasing evidence confirmed its unique role. Kisspeptins suppress metastasis by restricting the growth of the secondary tumour.(140) Binding of kisspeptin to KISS1R/Kiss1r increases intracellular calcium and activation of MAPKs, which limits cell motility and proliferation.(141) Kisspeptins have been investigated as potential treatment targets for melanoma,(142) thyroid cancer,(11) bladder cancer,(143) squamous cell carcinoma of the oesophagus,(144) gastric cancer,(145) hepatocellular carcinoma(146) and breast cancer.(138,147) Further studies may provide positive insights in this field.
POTENTIAL CLINICAL APPLICATIONS
Kisspeptin agonists and antagonists have potential diagnostic and therapeutic applications. Kisspeptin agonists may localise lesions in HPG axis dysfunction and can be used to evaluate the gonadotrophic potential of infertile individuals. They may also be used to treat subjects with subfertility through the stimulation of LH to result in ovulation. Kisspeptin antagonists may reveal the role of kisspeptins in various physiological and pathological states of the HPG axis.(25) As they reduce LH pulse frequency and amplitude without affecting basal LH secretion, kisspeptin antagonists may be useful as contraceptives in women or in the treatment of sex steroid-dependent diseases, such as prostate and breast cancer, endometriosis and uterine fibroids.(50,100) Furthermore, they may prevent premature luteinisation during in vitro fertilisation.(148,149) Lastly, kisspeptins may be used in the treatment of metastatic cancers.
CONCLUSION
Kisspeptins play essential roles in reproduction. They are involved in in utero sexual development and determine the onset of puberty. More importantly, they may be the link between energy homeostasis and the reproductive system. After sexual maturation, kisspeptins regulate HPG axis function by modulating gonadotrophin release. The difference in kisspeptin neuroanatomy accounts for sexual dimorphism of the HPG axis between genders. Potential clinical uses of kisspeptins include the treatment of delayed or precocious puberty, subfertility, downregulation of sex steroids in the treatment of sex steroid-dependent tumours, contraception and the treatment of metastatic cancers.





